The Physiology of Fat Loss
Fat may seem like the enemy of civilized people—especially sedentary ones. Yet we cannot live without it.
Fat plays a key role in the structure and flexibility of cell membranes, and it helps regulate the movement of substances through those membranes. Special types of fat, known as eicosanoids, send hormone-like signals that exert intricate control over many bodily systems, mostly those affecting inflammation or immune function.
Of course, the best-known function of fat is as an energy reserve. Fat has more than twice the energy-storage capacity of carbohydrate (9 calories per gram vs. 4 calories per gram). It has been estimated that lean adult men store about 131,000 calories in fat (Horowitz & Klein 2000), enough energy to keep the average person alive for about 65 days.
For fitness professionals, the prime concern arises when the body’s fat-storage function works too well, hoarding unwanted fat that makes people unhealthy and self-conscious about their appearance. Understanding how fat travels through the body can help personal trainers work with clients to reduce excess body fat and improve athletic performance.
The Journey of a Fatty Acid to Muscle
THE ADIPOCYTE
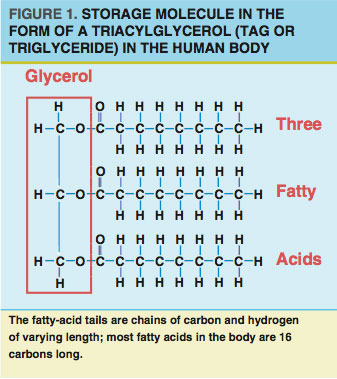
Fat resides primarily in designated fat-storage cells called adipocytes. Most adipocytes are just under the skin (subcutaneous fat) and in regions surrounding (and protecting) vital organs (visceral fat). Nearly all fat in adipocytes exists in the form of triacylglycerols (TAGs or triglycerides). Each TAG consists of a backbone (glycerol) with three fatty-acid tails (see Figure 1).
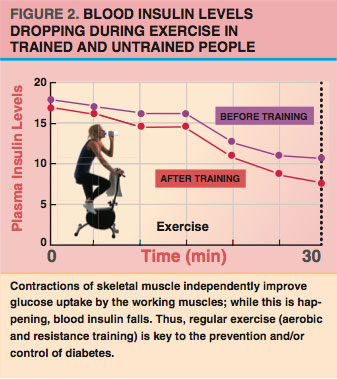
Depending on energy supply and demand, adipocytes can either store fat from the blood or release fat back to the blood. After we eat, when the energy supply is high, the hormone insulin keeps fatty acids inside the adipocytes (Duncan et al. 2007). After a few hours of fasting or (especially) during exercise, insulin levels tend to drop (see Figure 2), while levels of other hormones—such as epinephrine (adrenaline)—increase.
When epinephrine binds to adipocytes, TAG stores go through a process called lipolysis (Duncan et al. 2007), which separates fatty acids from their glycerol backbone. After lipolysis, fatty acids and glycerol can leave the adipocytes and enter the blood.
Fatty Acids in the Blood
Because fat does not easily dissolve in water, it needs a carrier protein to keep it evenly suspended in the water-based environment of the blood. The primary protein carrier for fat in the blood is albumin (Holloway et. al. 2008). One albumin protein can carry multiple fatty acids through the blood to muscle cells (Horowitz & Klein 2000). In the very small blood vessels (capillaries) surrounding the muscle, fatty acids can be removed from albumin and taken into the muscle (Holloway et al. 2008).
Fatty Acids Going From the Blood Into Muscle
Fatty acids must cross two barriers to get from the blood into the muscle. The first is the cell lining of the capillary (called the endothelium), and the second is the muscle-cell membrane (known as the sarcolemma). Fatty-acid movement across these barriers was once thought to be extremely rapid and unregulated (Holloway et al. 2008). More recent research has shown that this process is not nearly as fast as once thought and that the presence of special binding proteins is required at the endothelium and sarcolemma for fatty acids to pass through (Holloway et al. 2008). Two proteins that are important for fatty-acid transport into the muscle cells are FAT/CD36 and FABPpm.
Two Fates of Fat Inside Muscle
Once fat is inside the muscle, a molecule called coenzyme A (CoA) is added to the fatty acids (Holloway et al. 2008). CoA is a transport protein that maintains the inward flow of fatty acids entering the muscle and prepares the fatty acid for one of two fates:
- oxidation (in which electrons are removed from a molecule) to produce energy or
- storage within the muscle (Holloway et al. 2008; Shaw, Clark & Wagenmakers 2010)
The majority (80%) of fatty acids entering the muscle during exercise are oxidized for energy, while most fatty acids entering the muscle after a meal are repackaged into TAGs and stored in the muscle in lipid droplets (Shaw, Clark & Wagenmakers, 2010). Fatty acids stored in muscle are called intramyocellular triacylglycerols (IMTAGs) or intramuscular fat.
There are two to three times more IMTAGs stored in slow twitch muscle fibers (the slow oxidative fibers) than there are in fast-twitch muscle fibers (Shaw, Clark & Wagenmakers 2010). Shaw and colleagues note that even though this IMTAG supply makes up only a fraction (1%–2%) of the total fat stores within the body, it is of great interest to exercise physiologists because it is a metabolically active fatty-acid substrate especially used during periods of increased energy expenditure, such as endurance exercise.
Fatty Acids Burned for Energy
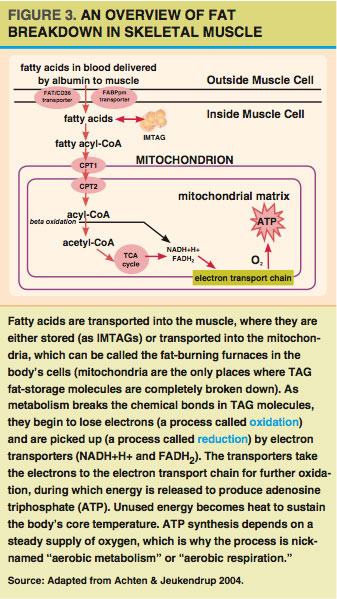
Fatty acids burned for energy (oxidized) in the muscle can come either directly from the blood or from IMTAG stores. For fatty acids to be oxidized, they must be transported into the cells’ mitochondria (see Figure 3). A mitochondrion is an organelle that functions like a cellular power plant; it processes fatty acids (and other fuels) to create a readily usable energy currency (ATP) in order to meet the energy needs of a muscle cell.
Most fatty acids are transported into the mitochondria via the carnitine shuttle (Holloway et al. 2008), which uses two enzymes and carnitine (an amino acid-like molecule) to do the transporting. One of these enzymes is called carnitine palmitoyltransferase I (CPT1). CPT1 may work with one of the same proteins (FAT/CD36) used to bring fatty acids into the muscle cells from the blood (Holloway et al. 2008). Once inside the mitochondria, fatty acids are broken down through several enzymatic pathways—including beta-oxidation, the tricarboxylic acid (TCA) cycle and the electron transport chain—to produce ATP.
Fatty-Acid Oxidation During a Single Bout of Exercise
At the start of exercise, more blood flows to adipose tissue and muscle (Horowitz & Klein 2000), releasing more fatty acids from adipose tissue and delivering more fatty acids to the muscle.
Exercise intensity has a great impact on fat oxidation.We burn the most fat when exercising at low to moderate intensity—that is, when oxygen consumption is between 25% and 60% of maximum (Horowitz & Klein 2000). At very low exercise intensities (25% VO2max), most of the fatty acids used during exercise come from the blood (Achten & Jeukendrup 2004). As exercise increases to moderate intensity (around 60% of VO2max), most of the fatty acids oxidized appear to come from IMTAG stores (Horowitz & Klein 2000).
At higher exercise intensities (>70% VO2max), total fat oxidation falls below the levels observed at moderate intensity (Horowitz & Klein 2000). This reduction in fatty-acid oxidation is coupled with an increase in carbohydrate breakdown to meet the energy demands of the exercise (Horowitz & Klein 2000).
We often overemphasize the fatty-acid contribution to calories burned during a bout of exercise. It’s also important to consider recovery from a bout of exercise, as well as training adaptations to repeated bouts, if you’re helping clients meet their fat-loss goals.
Energy and Fat Used During Recovery
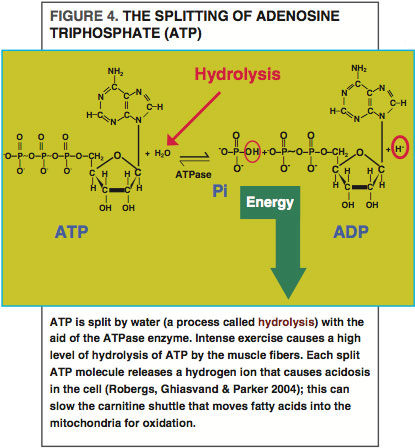
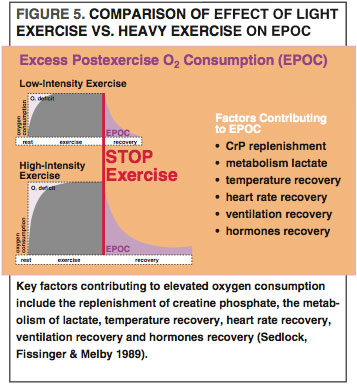
After we finish exercising, our body still needs to burn more energy, primarily to help muscle cells recover and to replace lost glycogen. This elevated metabolic rate is called excess postexercise oxygen consumption (EPOC), which appears to be greatest after high-intensity exercise (See Figure 5) (Sedlock, Fissinger & Melby 1989). For example, EPOC is higher after high-intensity interval training (HIIT) than after longer-duration, lower-intensity exercise (Zuhl & Kravitz 2012). EPOC is also notable after resistance training (Ormsbee et al. 2009), which disturbs the working muscle cells’ homeostasis to a great degree, meaning that more energy is needed to restore the contracting muscle cells to preexercise levels. EPOC stays particularly elevated for longer after eccentric exercise, as this activity creates a higher demand for cellular repair and protein synthesis (Hackney, Engels & Gretebeck 2008). Many studies also show that fat-oxidation rates rise during EPOC (Achten & Jeukendrup 2004; Jamurtas et al. 2004; Ormsbee et al. 2009). Comparatively, fatty-acid use during high-intensity bouts of exercise, such as HIIT and resistance training, may be lower than in moderate intensity endurance training; however, high-intensity exercise and weight training may make up for this deficit with increased fatty-acid oxidation through EPOC.
Adaptations to Exercise That Improve Fat Usage
Compared with their untrained counterparts, trained people can use more fat at both the same absolute (speed or power output) and the same relative (% of VO2max) exercise intensities (Achten & Jeukendrup 2004). Interestingly, lipolysis (the breakdown of fats to release fatty acids) and fat release from adipocytes are identical in untrained and trained people (Horowitz & Klein 2000). This suggests that trained people are better able to burn fat because of differences in the muscle’s ability to take up and use fatty acids, not because of the adipocytes’ ability to release fatty acids. Adaptations that enhance fat usage in trained muscle can either improve fatty-acid availability to the muscle and mitochondria or improve the ability to oxidize fatty acids.
Fatty-Acid Availability
Exercise causes specific proteins to deliver more fatty acid to the muscle and mitochondria. Exercise also increases the amount of FAT/CD36 on the muscle membrane and mitochondrial membrane (Holloway et al. 2008) and boosts CPT1 on the mitochondrial membrane (Horowitz & Klein 2000). Together, these proteins improve fat transport into the muscle and mitochondria to be used for energy.
Exercise may also cause changes in the intramuscular lipid droplets, which contain IMTAGs that usually reside near the mitochondria (Shaw, Clark & Wagenmakers 2010). The close proximity allows an efficient release of fatty acids from the lipid droplets to the mitochondria.
Exercise training also boosts IMTAG availability by causing the lipid droplets to conform more closely to the mitochondria. This increases surface area for more-rapid fatty-acid transport into the mitochondria (Shaw, Clark & Wagenmakers 2010). Exercise may also increase total IMTAG stores (Shaw, Clark & Wagenmakers 2010).
Another training adaptation that may improve fatty-acid availability is an increase in the number of small blood vessels within the muscle (Horowitz & Klein 2000). Remember, fatty acids can enter the muscle through the very small capillaries. Increasing the number of capillaries around the muscle enables increased fatty-acid delivery into the muscle.
Fatty-Acid Breakdown
IMTAGs are readily available for energy during exercise because they are already in the muscle. Trained athletes have an increased ability to use IMTAGs efficiently during exercise (Shaw, Clark & Wagenmakers 2010). Athletes also tend to have larger IMTAG stores than lean sedentary individuals. Overweight and obese people, interestingly, also have high levels of IMTAGs but cannot use them during exercise in the same ways athletes can (Shaw, Clark & Wagenmakers 2010).
So why are obese people less able to use IMTAGs? It’s not because their mitochondria cannot use fatty acid properly (Holloway et al. 2008). It’s probably because obese people have fewer mitochondria per unit of muscle—that is, they have low mitochondrial density (Holloway et al. 2008). Exercise increases mitochondrial density (Horowitz & Klein 2000; Zuhl & Kravitz 2012) and improves the ability to burn fat, which benefits people with fat-loss goals.
Endurance training effectively boosts the body’s ability to use fatty acids by improving the availability of fatty acids to the muscle and mitochondria and by increasing fatty-acid oxidation (Horowitz & Klein 2000). HIIT training results in similar fat-burning adaptations while requiring fewer workouts and less total time commitment (Zuhl & Kravitz 2012).
Practical Application
Rather than just trying to maximize fat oxidation in a single bout of exercise, personal trainers are better off designing workout programs aimed at causing muscle adaptations that improve their clients’ ability to oxidize fatty acids. Exercise professionals should include interval and endurance training programs, which can improve mitochondrial density and fat oxidation (Zuhl & Kravitz 2012). In addition, regular, progressively increasing programs of resistance training will enhance EPOC and postworkout fat oxidation. Trainers should encourage clients to engage in low- to moderate-intensity exercise (such as walking and cycling) on “off hard workout days” to enhance caloric deficits and support muscle adaptions between training days.
Mike Deyhle, CSCS, is a master’s student in exercise science at the University of New Mexico, Albuquerque. He is interested in neural and skeletal muscular physiology, especially with respect to skeletal muscular damage, metabolism, fatigue and exercise training/detraining.
Christine Mermier, PhD, is an assistant professor and exercise physiology laboratory director in the exercise science program at the University of New Mexico, Albuquerque. Her research interests include the effect of exercise in clinical patients, women and aging populations, and high-altitude physiology.
Len Kravitz, PhD, is the program coordinator of exercise science and a researcher at the University of New Mexico, Albuquerque, where he has won the Outstanding Teacher of the Year award. He was honored with the Can-Fit-Pro Lifetime Achievement Award in 2008 and received the 2010 Aquatic Exercise Association Global Award.
© 2014 by IDEA Health & Fitness Inc. All rights reserved. Reproduction without permission is strictly prohibited.